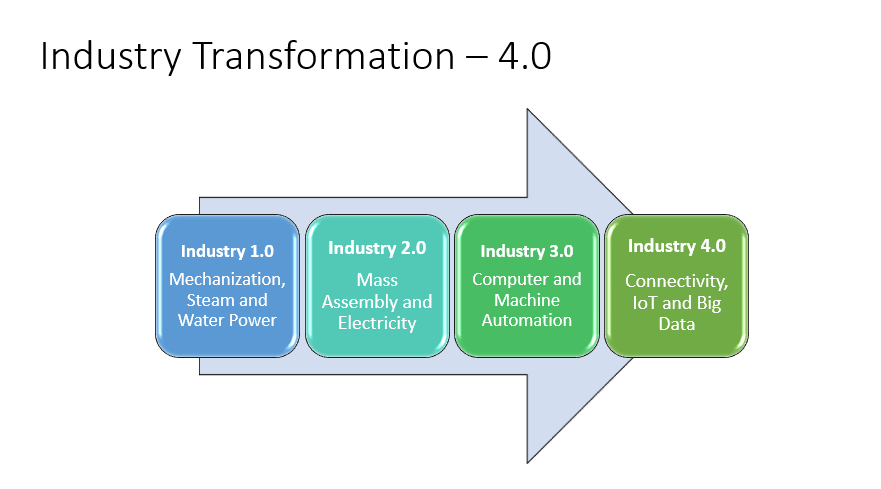
In this blog post, Sandra Acosta explains what changes are to come in the drug development field in 2023.
2022 Breakthroughs
Posted by
Sandra Acosta
What does the future hold for biotech and pharma? Find out in part 2 of this blog series.
EG Life Sciences explains the types of vendor audits that may happen in the pharmaceutical industry so programming teams can be prepared. Read more.
Dr. Terry Barnhart uses the example of Operation Warp Speed and COVID-19vaccines to show how we can speed up pharmaceutical innovation. Learn more today!
A director of product management shares what works and what doesn't when implementing Agile within drug discovery organizations. Learn more today.
Waterfall or Agile: Wrong Question!
Posted by
Bob Ellis
Follow Sandra Acosta, Director of Biopharma Solutions, as she considers the difference between biotech and pharma and what's next for these industries.
Scientist and Agilist Kendra West shares tips for introducing Agile practices within scientific project management. Learn from her findings today.
Dr. Terry Barnhart shares how your company can boost innovation through Agile pharmaceuticals. Start accelerating your innovation today.
Have you ever wondered what goes into the production of viral vector vaccines? Read on.
In this blog post, Sandra Acosta answers timely questions in the life sciences space: What are viral vectors? And how do the types of viral vectors work?
With new technologies like cell gene therapy and viral vectors, we have a better chance of beating COVID-19 and serious diseases. Learn more here.
Eliassen Group experts talk about the impact of automation on continuous compliance, which can lead to savings and an improvement in outcomes.
Sandra Acosta shares the steps that industries can follow to improve safety within the vaccine industry. Read more.