As shared in our previous blog post, "Cell Gene Therapy and Viral Vectors," the making of a vaccine is a complex process that requires extensive knowledge and expertise to minimize all potential risks that could be a threat to human life. The vaccine industry used to be relatively small, but due to recent events, there is more demand for expertise in the field.
Most companies not only have implemented effective and efficient processes and technology, but they also use highly skilled professionals who are working to leverage and continue improving processes to maintain product quality. Elaboration of a vaccine takes several steps and manipulations under a very strict classified environment, which includes several critical controls like temperature and humidity, among others, requiring maintaining conditions to continuously produce a pure and safe product.
Let’s continue this series expanding on one of the most popular vaccine manufacturing applications — Viral Vectors, their contribution toward eradicating COVID-19, and the experts behind the scenes.
The Production of Viral Vectors for Vaccines
The production of viral vectors for vaccines requires a high level of biosecurity. Therefore, low pathogenic viruses are often selected. When using viral vectors, it is important to assess the potential effects by understanding epidemiological and virological characteristics. The specific property of the vector is determined by the virus, and each vector has advantages and disadvantages. Adenovirus are the most widely used vectors because they can induce a strong immune response against the expressed foreign antigens.
This process requires state-of-the-art technology and strict control following Good Manufacturing Practices (GMP) guidelines. To maintain high quality and a safe product, this process is campaign-based, a single product is produced at a time, and disposable materials and closed systems are often used. Open processes are executed in biosafety cabinets with HEPA filters, with air exhaust directly out of the manufacturing facility.
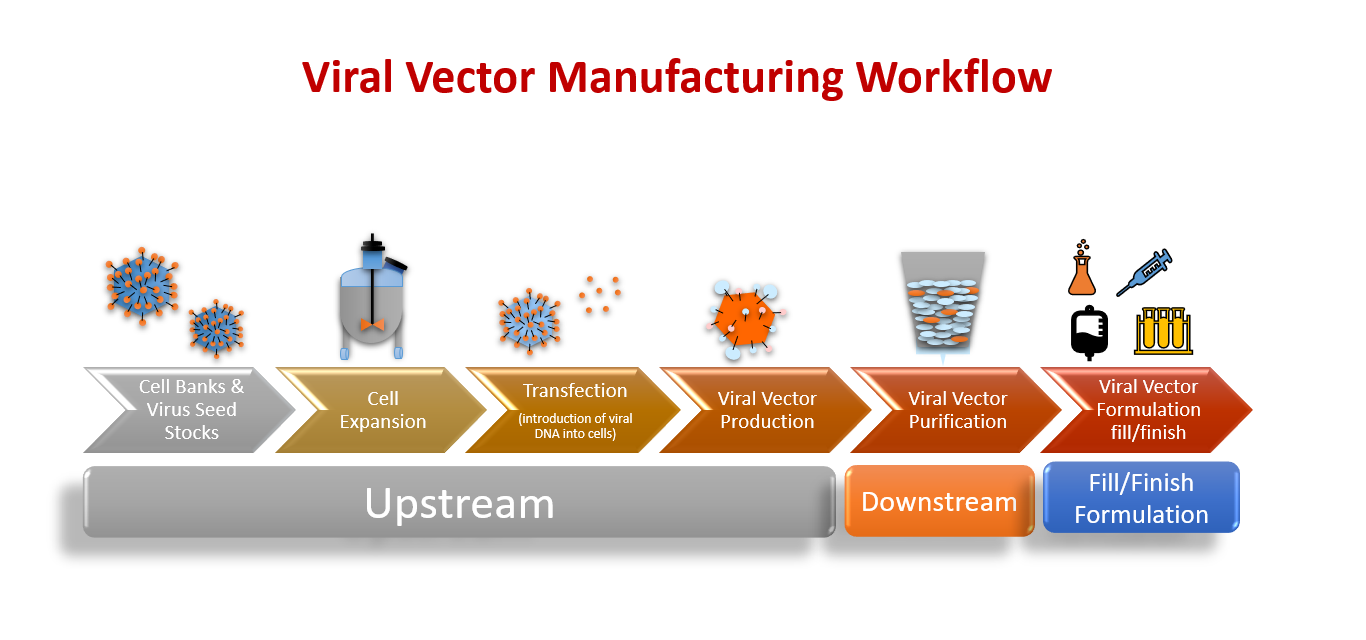 Figure 1: The Viral Vector Manufacturing Workflow, including Upstream Processes, Downstream Processes, and Fill/Finish Formulation.
Figure 1: The Viral Vector Manufacturing Workflow, including Upstream Processes, Downstream Processes, and Fill/Finish Formulation.
Viral Vector Production Suite Area
The production suite area is equipped with HVAC technology with a single-pass ventilation design. The facility includes a restricted passage with electronic access control and pressured airlocks to control material and personnel entry and exit. HEPA filters are placed across the operating area and airlocks. Other utilities include gas supplies (CO2, O2, N2) and compressed air outlets. The facility requires 24/7 monitoring and an alarm system due to the sensitivity and process security.
Different viruses require slightly different production processes, meaning separate equipment and facilities are needed for each one. Everything starts on a small scale at the lab, a process that may take more than 20 scientist interventions to ensure master cells are ready to go to the production process. Once large amounts of virus or bacteria have been grown, they must then be isolated, purified, and attenuated or inactivated, depending on the vaccine. Each of these steps requires specific equipment, reagents, and stringent procedures to avoid, and check for, contamination, which can further increase costs.
For example, polio virus is grown in dishes of cells, which require different handling, while bacteria-based vaccines are grown in vast bioreactors. Growing live pathogens also means stringent precautions must be taken to avoid the virus escaping and making vaccine plant operators sick.
Process
Once the bad portion of the virus is removed from the capsule, scientists map the genetic material for the specific treatment to be delivered to the host/cell tissue. The master viral vector is then ready for large-scale vaccine production for formulation and vial fill finish process. The formulation is determined finding the right vaccine volume in mL (cc) to be administered to patients. This genomic isolation can be also utilized for cell-gene therapy applications.
As we learned from "What Are Viral Vectors and How Do They Work?" most Contract Manufacturing Operations (CMOs) and Contract Development Manufacturing Operations (CDMOs) facilities manufacture vaccines on a large scale, and that upstream and downstream processes are the most critical steps during production.
Below, we see a high-level summary of the viral vector vaccine production process:
Lab — Working Cell Bank
- Frozen viral master cell bank and seed stock
- Suspension Cell Culture in single-use bioreactors — up to 200 L
- Adherent Cell Culture in multilayer flasks, packed-bed bioreactor, and single-use bioreactors
- Ultracentrifugation
- Chromatography
- Purification Solutions — chromatography, membrane, and Tangential Flow Filtration (TFF)
- Formulation and final Drug Product manufacturing
- Automated Aseptic Filling line for live Viral Vectors
- Typical batch sizes 200–1, 000 vials
- Specific Assays
- Impurity Analysis
- Cell-Based Assays
- Microbiological QC and Safety Assays
- Release
The following images illustrate both upstream and downstream processes. Upstream Processes include the thawing of cells, cell expansion, infection with virus seed or transfection with plasmids, and culturing of the Viral Vector producing cells.
Figure 2: A visual of all Upstream Processes.
Some scientists say that the cell lysis and clarification steps are transitional steps that links the Up- and Downstream Processes, and they are sometimes called Midstream Processes. Downstream Processes exist to enrich the Viral Vector product while eliminating contaminants that originate from host cells and the culture medium.
Figure 3: A visual of all Downstream Processes.
Advantages and Disadvantages of Most Popular Viral Vectors
Viral vector-based vaccines present advantages over traditional vaccines in that they can enhance a broad range of immunogenicity; however, there are some disadvantages of major viral vectors utilized in the modern vaccine industry.
General Advantages of Viral Vector-Based Vaccines
- High-efficiency gene transduction
- Highly specific delivery of genes to target cells
- Induction of both humoral and cell-mediated immune responses
- Reduced administration doses
- Enable large-scale manufacturing
- Potential targets ranging from cancers to a vast number of infectious diseases
General Disadvantages of Viral Vector-Based Vaccines
- Risk of integration into the host genome, leading to other diseases
- The presence of pre-existing immunity against the vector caused by previous exposure to the virus and the production of neutralizing antibodies can reduce vaccine efficacy
Figure 4: Advantages and disadvantages of major viral vectors by specific virus type.
Challenges in the Production of Viral Vectors
A major bottleneck for viral vector vaccine production is scalability. Traditionally, viral vectors are grown in cells that are attached to a substrate, rather than in free-floating cells, but this is difficult to do on a large scale. Suspension cell lines are now being developed, which would enable viral vectors to be grown in large bioreactors.
Assembling the vector vaccine is also a complex process, involving multiple steps and components, each of which increases the risk of contamination. Extensive testing is therefore required after every step.
In summary, several viral vectors have been successfully used for vaccine production and gene therapy. These vaccines can potentially induce a strong immune response in tissues and cells and achieve targeted delivery. Early-phase trials have shown that they are well tolerated in humans. Although additional challenges lie ahead, the prospect of viral vector vaccine looks promising.
There are more advantages than disadvantages in the making of vaccines using viruses. Research and development is ramping up in the clinical trial phases and providing a significant collaboration for the medicine of the future.
Stay tuned for my next posts, which will cover the following subjects:
- The other advantages and disadvantages of live attenuated vaccines, inactivated virus vaccines, and the different top COVID-19 vaccines currently being used
- Specific process controls and the promising future of the viral vector technology, including the capabilities and skills that experts require to avoid cross-contamination in vaccine production
Image Sources
All images created by Sandra Acosta
Sources
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/viralvector.html Accessed 15 June 2021.
https://www.who.int/biologicals/areas/vaccines/Annex_2_WHO_Goodmanufacturing_practices_for_biological_products.pdf Accessed 15 June 2021.
https://ichgcp.net/ Accessed 15 June 2021.
https://www.fda.gov/vaccines-blood-biologics/biologics-guidances/cellular-gene-therapy-guidances Accessed 15 June 2021.

