As more vaccines for COVID-19 emerge, safety in the vaccine production process is crucial. Any foreign matter entering the vaccine production process raises major safety concerns, and contamination is a common occurrence and a public health risk.
All of us who work in the Biotech industry or the pharmaceutical industry, including those of us at EG Life Sciences, face daily challenges to maintain an aseptic environment. Rigorous safety standards are in place to avoid any cross-contamination and/or any foreign matter being introduced to the vaccine production process. However, there is still a chance that this could happen.
Many potential particulates and contaminants could significantly impact the aseptic environment for any manufacturing product process, including those for vaccines, injections, IV solutions, etc. In addition to the toxic substances deliberately added to vaccines, such as aluminum, mercury, MSG, and Polysorbate 80, etc., products like vaccines are also frequently contaminated by viruses, bacteria, mycoplasma, metals, and dangerous chemicals. In fact, due to the materials used and the manufacturing process itself, contamination is almost inevitable.
How Cross-Contamination Happens in Vaccine Production
Cross-contamination occurs when particulates of one product or ingredient are inadvertently mixed with another. This can happen when the same equipment and/or components are used to process multiple products and are not adequately cleaned prior to mixing new solutions, or when multiple product lines are operating near one another. Because different drugs interact differently, cross-contamination will negatively impact the drug’s efficacy, cause other health problems, trigger an allergic reaction to patients, or even be fatal. Even contamination via particle buildup from a single substance can cause problems by altering the intended dosage of the product throughout the entire formulation.
There are also three main types of contamination to consider in pharmaceutical manufacturing and transportation of items and materials into sterile controlled areas:
- Physical
- Chemical
- Viable Microbial
Physical Contaminants
Physical contaminants are mostly visible particles coming from raw materials and/or shipped components. These could be metal, glass, or fibers. In addition, dust, dirt, or rust can potentially be introduced inside controlled clean suites. All these contaminants could cause adverse effects if they get in contact with manufacturing equipment surfaces, product excipients or inactive substances, product contact surfaces, and/or the room environment. Particulates in oral medications could wear away at internal organs or pose a toxicological threat, with lead and chromium being the most dangerous in terms of poisoning potential. A thorough cleaning process can help prevent such materials from being introduced to clean, controlled areas.
Chemical Contaminants
These contaminants are unwanted chemicals that are not part of the drug. Some sources of contamination in pharmaceutical manufacturing could be through drug intermediates, excipients, and cleaning reagents; however, most of the chemical contamination sources found in the vaccine industry come from the biological itself.
In the vaccine world, viruses, animal cells, and other synthetic sources are utilized to make the final product. Most difficult to handle are the viruses. In theory, one single infectious virus particle may be sufficient to initiate an infection that could have potentially dangerous, or even fatal, consequences in humans, and this is especially true in vulnerable patients, whose immune systems could already be compromised. Even viruses that are not known to cause significant disease in humans could, potentially, do so if introduced by a non-natural route of infection, such as by injection rather than respiratory or oral spread. Contamination with retroviruses is a particular concern because retroviruses integrate into the host cell genome as part of their lifecycle and can result in life-long infection or cellular change.
Virus contamination could also arise from the presence of a virus in the original cell line or biological system used, or from the inadvertent introduction of a virus into cell cultures during a manufacturing process. Potential contamination sources could include raw materials of animal origin, the human operator, or materials contaminated by contact with animals or animal-derived material. For example, contamination of Chinese hamster ovary (CHO) cell banks with murine minute virus (MMV)—a virus primarily transmitted in the urine of rats and mice—could, potentially, have resulted from this latter route.
Viable Microbial Contaminants
When a medication is contaminated with microorganisms like bacteria or fungus, the consequences can range from harmless to fatal. For nonsterile drugs taken orally, the effects may be less likely to be dire, as our gastrointestinal tracts provide a highly acidic, unhospitable environment that can kill most microorganisms. However, medications that are administered intravenously or in the form of eye drops must be completely sterile. Any contaminant can find its way directly into the bloodstream, causing sepsis or even death.
Other Sources of Contamination in Pharmaceutical Manufacturing
Even though production takes place within a control environment, chances are that any cross-contamination can happen from the production environment to the product. The production environment includes air, surfaces, instruments, equipment, and personnel. A clean room is a place with high control of the entrance of particles via the establishment of some air filters called high-efficiency particulate air (HEPA) filters. HEPA filters made from a microglass material with a pleated construction system provide a large surface area that efficiently filters the incoming air and generates a constant air motion. Based on the permitted quantity of viable microorganisms and particulates, various classes of clean rooms can be established using HEPA filters with required efficiencies.
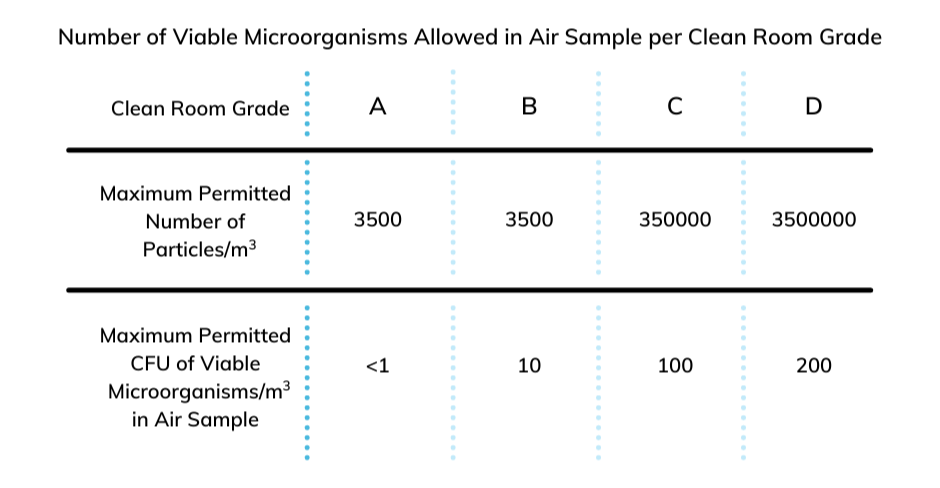
Figure 1 – Table of established standards showing just how many viable microorganisms are allowed in an air sample to achieve a specific Clean Room Grade. Note that you must have fewer than 1 colony-forming unit (CFU) of viable microorganisms in an air sample to achieve a Clean Room Grade of A.1
In addition to the factors mentioned above, other potential factors contribute to cross-contamination in the aseptic environment:
Personnel
- Lack of training
- Direct contact between operator hands and starting material, plus primary and intermediate product
- Storage
- Controlled areas
- Malpractices
- Inadequate gowning
Other Potential Causes of Cross-Contamination in Vaccine Production
- Lack of science and risk base cleaning limits
- Encourage disposal and dedicated product contact parts
- Poor cleaning procedure design
- Removal of unnecessary equipment
- Poor cleaning effectiveness
.png?width=940&name=Copy%20of%20Copy%20of%20Copy%20of%20Eliassen%20Group%20Highlights%20Expanding%20Service%20Offerings%20through%20Brand%20Evolution%20(1).png)
Figure 2- Common causes of contamination measured by Current Good Manufacturing Practices (cGMP) and non-cGMP positive and negative material2
Summary
So, we have learned for years in this practice that cross-contamination is a potential risk for all aseptic environments. These contaminants can come from any source, particularly from same cell line strains, individuals during manipulations, materials transfers, equipment, setups, etc., and laboratory practices where all cell/virus line are prepared.
On the other hand, these contaminations can be reduced significantly not only through rigorous safety practices, but also through additional technology added to the process. Some contaminants, however, like those coming from living cell lines (especially viruses, which are more difficult to handle), cannot be eliminated on a 100% basis, as this will become part of the same vaccine and same product.
Expertise and good practices are the key to minimizing the impact of contaminants in aseptic environments and products. There are a few important factors to consider in the manufacturing facility:
- Cleaning techniques
- Substances to remove from equipment
a. Carry over from other products and contaminants - Analytical techniques for cleaning verification
- Designing equipment-specific procedures
- Maintaining a robust cleaning program
Three principal and complementary approaches are generally applied for controlling viral contaminants in biologics. Read my follow-up blog post to learn more about viral controls and prevention of cross-contamination in the vaccine industry.
If you feel you need support in this area, EGLS has the expertise to help you improve your vaccine production processes and reduce risk.
Image Sources
- Lotfipour, Farzaneh, and Somayeh Hallaj-Nezhadi, “Microbial Quality Concerns for Biopharmaceuticals,” December 12, 2012, https:/www.intechopen.com/books/latest-research-into-quality-control/microbial-quality-concerns-for-biopharmaceuticals. Accessed 3 March 2021.
- Barone, Paul W., and Michael E. Wiebe. “Viral Contamination in Biologic Manufacture and Implications for Emerging Therapies.” Nature News. Nature Publishing Group, April 27, 2020. https://www.nature.com/articles/s41587-020-0507-2. Accessed 3 March 2021.
Other Sources