Many of you reading this blog have asked yourselves, "What does the future in biotech and pharma look like?" When I started thinking about the answer to this question, I remembered the Dick Tracy comic strip in which the hero wore an electronic gadget on his wrist, a 2-way wrist radio. Apple came out with their invention of the Apple Watch as the first smart watch on April 24, 2015, wowing consumers everywhere. However, Chester Gould, the creator of Dick Tracy, had the idea first when he debuted the iconic 2-way gadget on January 13, 1946.
So, even though Apple broke new ground with their smart watch, Chester Gould was already ahead of them. What we consider to be "the future" is happening right now, or even in the past, especially in the life sciences, which represent a highly dynamic space of scientific advancements.
"My only thought is to keep my strip faithfully realistic and powerful enough so that it will stand out from the usual run of wishy-washy everyday stuff." -- Chester Gould, creator of the Dick Tracy character
Biotech and pharmaceutical companies are classifying diseases in categories and patient groups for drug discovery and development. This will bring tremendous growth and more effective drugs to market targeting those specific patient groups. Holistic lean manufacturing will become more popular, eventually turning into a standard practice. Having faster response times aligned with high market demands will require less warehouse stock or even zero stock, as expected in some designs. Treatments will be more direct to the patient and personalized; this will provide flexibility on drug batch size requirements. Some therapies will be made to order, not to forecast.
All these innovations are possible through new tools and technologies, along with all clinical discoveries and relevant data that have been accumulated throughout the years. Yet we are continuously asking questions of the future. Where will we be in the coming years? Are we moving from a healthcare system that treats people after they’re sick to one designed on preventive and diagnostic medicine? Will we treat patients for conditions and diseases we already know or those that we will know in advance they are likely to develop?
Join me to find out more about the future in the biotech/pharma world, how these industries relate to past innovations, and the extraordinary opportunities ahead.
What Is the Difference Between Biotech and Pharma?
To understand the difference between the biotechnology and pharmaceutical manufacturing industries better, I would like to walk through their applications. Both industries manufacture medicine; however, the biotech industry produces medicines from living organisms, while the pharmaceutical industry produces medicines based on chemicals. And then you have biopharma, which combines both biotechnology and chemicals in its research and development processes. To summarize:
- Biotechnology companies derive their medicines from living organisms
- Pharmaceutical companies produce medicines from chemicals and/or synthetic processes
Biotechnology
A variety of manufacturing processes exist in biotechnology. They can be applied to agricultural products, beer, wine, detergents, and medical applications. The use of DNA in biotechnology has contributed tremendously to help this industry make great advancements. Some of the technologies utilized in biotechnology are:
- Nanobiotechnology
- Tissue engineering and regeneration
- DNA sequencing
- Cell-based assays
- Fermentation
- Chromatography
There is also an extensive variety of products within biopharma that provide alternatives to treat autoimmune diseases like arthritis with the production of the injectable HUMIRA, which was initially launched by Abbott.
Biologics and Biosimilars
Before I speak more about pharmaceuticals, I would like to also share the differences between biologics and biosimilars.
Biologics therapies include a wide range of medical products. A few of them from the first generation of biologic therapies include vaccines, blood products, and stem cell injections. However, when we refer to second-generation biologic therapy drugs, we refer to manufactured drugs like HUMIRA, Remicade, or Enbrel. These drugs cannot be made using a simple chemical reaction, such as mixing ingredients prepared in a laboratory; instead, biologic therapies are made using living organisms, such as bacteria, yeast, and even mammalian tissue and cells. Biologic drugs are made using a highly complex manufacturing process known as Biotechnology, which we will cover in the future.
Biosimilars are similar to, but not identical to, the original medicine, while biologics are large, complex proteins made from living cells through highly complex manufacturing. So, a biosimilar product is approved based on demonstrating that it is highly similar to an FDA-approved biologic product, known as a reference product, and it does not have clinically meaningful differences in terms of safety and effectiveness from the reference product. Biosimilars are inherently different from generics due to their molecular size and structure, and the complexity and cost of their development, and they also have significantly higher research and development costs and risks that make them more complex to manufacture than small molecule generics.
Pharmaceuticals
The pharmaceutical industry is also undergoing rapid growth. This high-profit sector reaches annual sales in billions. However, new drug development can take many years and development phases in addition to regulatory agencies' testing and evaluation before a drug makes it to market. Even if a company develops a new drug for the market, it doesn’t mean it will get widespread physician approval and use. Usually, the pharmaceutical sector tries to keep several products in different stages of development, since new drug development could take 15 years through completion. Despite that, this sector provides stable results for especially large companies, and new companies appear frequently.
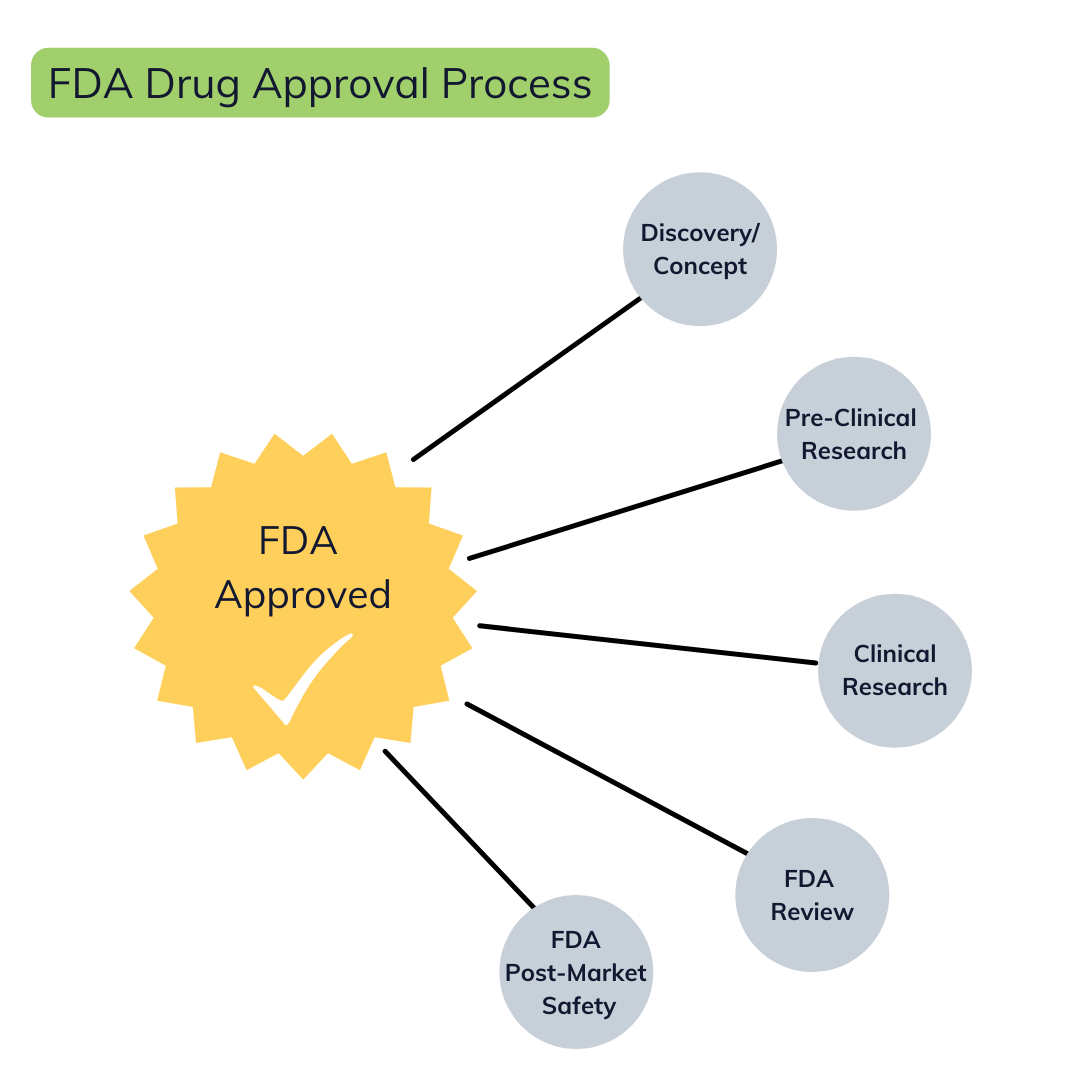
All the steps it takes for a drug to be approved by the FDA.
Recently, many pharmaceutical industries have adopted biotech because of the low cost of biotech's products. The costs for pharmaceuticals are often very high, and the results are better when combining pharmaceuticals and biotech due to biotech's sophisticated techniques and advanced technologies.
Pharma/Biotech Trends
After the COVID-19 outbreak, infectious diseases are driving the biotech/pharma market, especially the biotech sector. Companies are focused on new drug development so they can be prepared for any unexpected outbreak. The expectations are that manufacturing systems will be able to deliver more diverse and challenging products. The technology and skillsets to manufacture these novel medicines and therapies will become more complex, requiring flexibility and large investments. Drastic changes like changes in facilities and manufacturing methodologies are urgently required to be able to implement a flexible, productive, and efficient operation. Drug manufacturing processes, technologies, and facilities will also transform toward a future that is agile, flexible, and universal.
Globally the biotech market is expected to reach $2.44 trillion by 2028.
Driving New Technologies
Many manufacturing sites are increasingly utilizing modular building strategies. Facilities are including disposable process equipment, clean suites around process equipment, lean design concepts, and close system operation from seed to unit dose to minimize product adulteration and cross-contamination. Most of these changes significantly reduce cost and startup timeframes for new manufacturing systems and facilities.
Another concept that is very important to reducing cost, increasing efficiency, and assuring compliance is the Quality by Design (QbD) concept. This will provide significant product progress from Phase I to Phase III. Traditionally, this requires a large investment in a facility for a product to be manufactured, hoping that it will be successful in Phase III. Even though dedicated manufacturing facilities will be still required under certain conditions, agile and adaptable facility designs will become more widespread.
As a top priority, product quality is continuously on the regulatory agencies' radar; however, today’s regulatory environment also provides traction for the ongoing advancement of innovation. Authorities in the three ICH (International Council for Harmonization) regions and beyond are encouraging the biotech/pharma industry to adopt new technology along with the introduction of QbD concepts. This will lead the biotech/pharma industry to adopt cleaner, more flexible, and more efficient close systems.
Other technology drivers include the following:
- Scalability: Continuous manufacturing improves scalability, reduces time to market, and enhances quality while reducing capital and operating costs
- Process Analytical Tools: Streamline and strengthen processes, accelerate production scale-up, and ensure resources are efficient
- Alternate Downstream Process: Increase yields while reducing costs
- Green Chemistry: Reduce waste
- Capacity, Scalability, and Flexibility: Improve processes for new vaccine and therapy production methods
- Drug Device Combinations or Drug Delivery Systems: Increase the effectiveness of medicines and patient compliance
These evolutions toward the future on manufacturing techniques are not limited to facilities but apply throughout the supply network, and they will affect pharma/biotech companies in many ways:
- The need for specialized resources or SMEs with relevant skills to design programs and implement changes will rise.
- Diagnostics developers, as well as production equipment and medical device manufacturers not limited to academic institutions, will collaborate on manufacturing innovation.
- Business ecosystem advantages and location in strategic manufacturing decisions because of the new portfolios and technologies required to produce them.
To be successful, all collaboration and strategy should be focused on:
- Accelerating change
- Dynamic response
- Globalization
- Markets
- Competition
Conclusion
In summary, the future looks bright. Biotech/pharma companies have become the leading industry for therapy and multiple disease treatment. Molecular science capabilities, new technologies, R&D, new concepts, and the optimization of manufacturing processes have significantly contributed to drug development and effective treatments worldwide.
The growth expectations of biotechnology markets are high. The factors driving the market include favorable government policies, the launch of new and advanced drugs, robust investment in the biotech/pharma sector, and rising demand for synthetic biology. Nanotechnology will be increasing in the research and development sector. Agile concepts will be transforming not only R&D but also the biotech/pharma manufacturing companies to help them transform their functions in many applications.
Join me in my next blog, The Future of Biotech and Pharma Manufacturing, Part 2 to learn more about what to expect from biotech and pharmaceutical industry innovation and how Agile concepts can transform a traditional regulated industry to be a more flexible and faster operation.
If you feel you need support with your biotech or pharma projects, EG Life Sciences has the expertise to help.
Reach out to EG Life Sciences today!
Other Sources
- "Biological Risk Assessment: General Considerations for Laboratories." Centers for Disease Control and Prevention. Accessed 15 March 2022. https://www.cdc.gov/safelabs/resources-tools/bio-risk-assessment.html.
- "Biomedical Researchers." Centers for Disease Control and Prevention. Accessed 4 March 2022. https://phil.cdc.gov/group_researchers.aspx.
- "Biotechnology market size worth $2.44 trillion by 2028." Grand View Research. Accessed 4 March 2022. https://www.grandviewresearch.com/press-release/global-biotechnology-market.
- Chase, Lauren. “About the Philosophical Gourmet Report.” GoodRx Health. January 3, 2020. https://www.goodrx.com/healthcare-access/medication-education/what-are-biologics-and-biosimilars.
- "Process of Harmonisation." ICH. Accessed 4 March 2022. https://www.ich.org/page/process-harmonisation.


